One of the barriers to efficient second generation biofuels is creating a better way to break down the lignin in plants that is then converted to the sugars that create the building blocks of biobased products such as cellulosic ethanol, biomaterials and biochemicals. But this hurdle may be getting lower with research out of Lawrence Berkeley National Laboratory. Scientists have demonstrated an enzyme that can be tweaked to reduce lignin in plants.
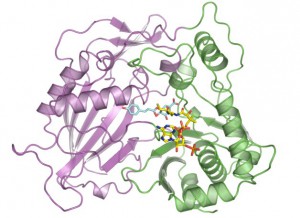
This illustration shows the molecular structure of HCT that was derived at Berkeley Lab’s Advanced Light Source. The purple and green areas are two domains of the enzyme, and the multi-colored structures between the two domains are two molecules (p-coumaryl-shikimate and HS-CoA) in the binding site. New research shows this binding site is indiscriminate with the acceptor molecules it recruits, including molecules that inhibit lignin production. (Credit: Berkeley Lab)
Lignin is essential to plant health. It resides in a plant’s cell walls and surrounds and traps the sugars inside. In order to extract the sugars, the lignin must first be broken down through chemical pretreament. Thus, the less lignin there is, the less expensive the pretreatment step becomes.
The research was published in Plant & Cell Physiology and focuses on an enzyme called HCT that plays a key role in synthesizing lignin in plants and has been found to be indiscriminate with what molecules it binds with. With this discovery, the researchers introduced another molecule to the enzyme that occupies the binding site usually occupied by the lignin-producing molecule. This swap inhibits the enzyme’s ability to support lignin production. Initial tests showed a decrease in lignin content by 30 percent while increasing sugar production, without weakening the plant.
“Our goal is to tune the process so that lignin is reduced in a plant where we want it reduced, such as in tissues that produce thick cell walls, and when we want it reduced, such as later in a plant’s development,” said Dominique Loque, a plant biologist with the Joint BioEnergy Institute (JBEI), a DOE Bioenergy Research Center led by Berkeley Lab, which pursues breakthroughs in the production of cellulosic biofuels. “This would result in robust bioenergy crops with more sugar and less lignin, and dramatically cheaper pretreatment costs.”
Next the researchers want to learn how to adjust the temporal and spatial specificity of the enzyme’s lignin-reduction abilities in plants. They also want to further study the Advanced Light Source-derived enzyme structures to see if HCT can be modified to be even more attractive to the new molecules.